|
|
TODAY.AZ / World news
WHO prequalifies first vaccine against mpox
13 September 2024 [23:30] - TODAY.AZ
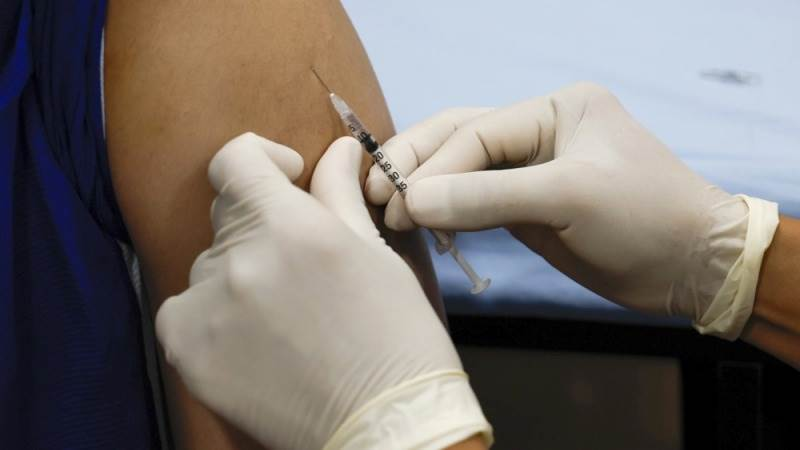
The World Health Organization (WHO) announced on Friday that it's prequalifying the MVA-BN vaccine made by Bavarian Nordic A/S as the first vaccine against mpox, Azernews reports.
"This first prequalification of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa, and in future," WHO Director-General Dr. Tedros Adhanom Ghebreyesus stated and added that an "urgent scale up in procurement, donations and rollout" is required for the vaccines to be available where they are needed the most.
According to the WHO's press release, the MVA-BN vaccine can be administered as a two-dose injection, with four weeks between the two doses. WHO stated that the vaccine is currently licensed only for people who are at least 18 years old, but it may be used "off-label" for younger persons in outbreak settings where the benefits of vaccination outweigh the potential risks.
URL: http://www.today.az/news/regions/252887.html
 Print version
Print version
Connect with us. Get latest news and updates.
See Also
- 15 November 2024 [23:30]
United States deploys naval reconnaissance aircraft in Scotland - 15 November 2024 [22:27]
Schumacher's latest car is up for auction - 15 November 2024 [21:46]
Tbilisi City Hall allocated 8.6 million lari for New Year's events - 15 November 2024 [20:48]
Iran prepares to treat women who violate hijab law - 14 November 2024 [23:45]
Minister Bayraktar emphasizes Turkiye's role in net zero emissions - 14 November 2024 [23:26]
Poland deployed South Korean K2 tanks near border with Russia - 14 November 2024 [22:50]
Pentagon spent $1 billion on the US military industry - 14 November 2024 [21:40]
US budget deficit quadrupled in year - 14 November 2024 [20:19]
Impressive warehouse discovered in Turkiye - 14 November 2024 [18:48]
Armed robot dog 'BARS' shows off in sniper competition
Most Popular
 Provocateur Le Pen go to jail
Provocateur Le Pen go to jail
 Azerbaijan's Leadership at COP29: Addressing Global Climate Challenges and Exposing Hypocrisy
Azerbaijan's Leadership at COP29: Addressing Global Climate Challenges and Exposing Hypocrisy
 Macron Stung by President Ilham Aliyev's Truth: French Minister Ordered to Skip Baku Visit
Macron Stung by President Ilham Aliyev's Truth: French Minister Ordered to Skip Baku Visit
 Who uses Greta Thunberg and how?
Who uses Greta Thunberg and how?
 Climate Platform for business, investment & philanthropy launched within COP29
Climate Platform for business, investment & philanthropy launched within COP29
 United States announced plans to deepen security cooperation with Indonesia
United States announced plans to deepen security cooperation with Indonesia
 Azerbaijan’s COP29 presidency sets precedent with focus on amplifying voices of vulnerable nations
Azerbaijan’s COP29 presidency sets precedent with focus on amplifying voices of vulnerable nations
